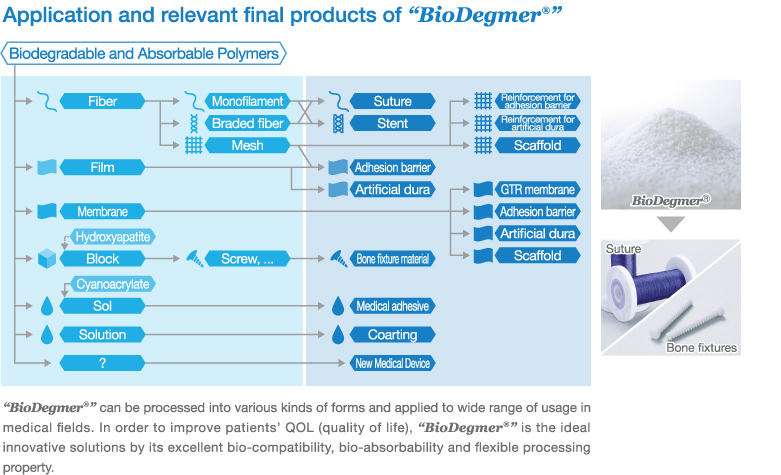
In 1986, through research collaboration with BMG, Gunze Ltd. obtained approval from Ministry of Health and Welfare, Japan for producing the first domestically produced bio-absorbable surgical sutures. This triggered BMG production of medical-use bio-absorbable polymer (PGA) for surgical sutures, for the first time domestically.
Thereafter, BMG had stepped forward to PGA production in larger quantities and also have been actively engaged in development and production of various types of bio-absorbable polymer materials. In 1996, production of Poly-L-lactide (PLLA) materials for bio-absorbable bone fixtures was commenced. In 1997, production of Poly (L-lactide-
co-
ε-caprolactone) (LCL) materials for bio-absorbable monofilament surgical sutures was commenced.
Nowadays, all of these biodegradable and absorbable polymer materials are produced in accordance with ISO 13485 and are supplied globally by the brand name of “
BioDegmer®”